Case Study II Biomedical System for Delivery of Stem Cells
Biomedical Devices for Neurotherapeutics
Background
Medrad (Bayer) was asked by Schering AG, to conceptualize, investigate and propose a means to wash the hibernation fluid from the neurological pharmaceutical Spheramine (a stem cell therapy intended to treat Parkinson’s disease).
There were many challenges to this product not only because we were delivering a therapeutic fluid to a human brain, but also the delicacy in which the suspension fluid had to be handled and washed due to the fragile nature of stem cells.
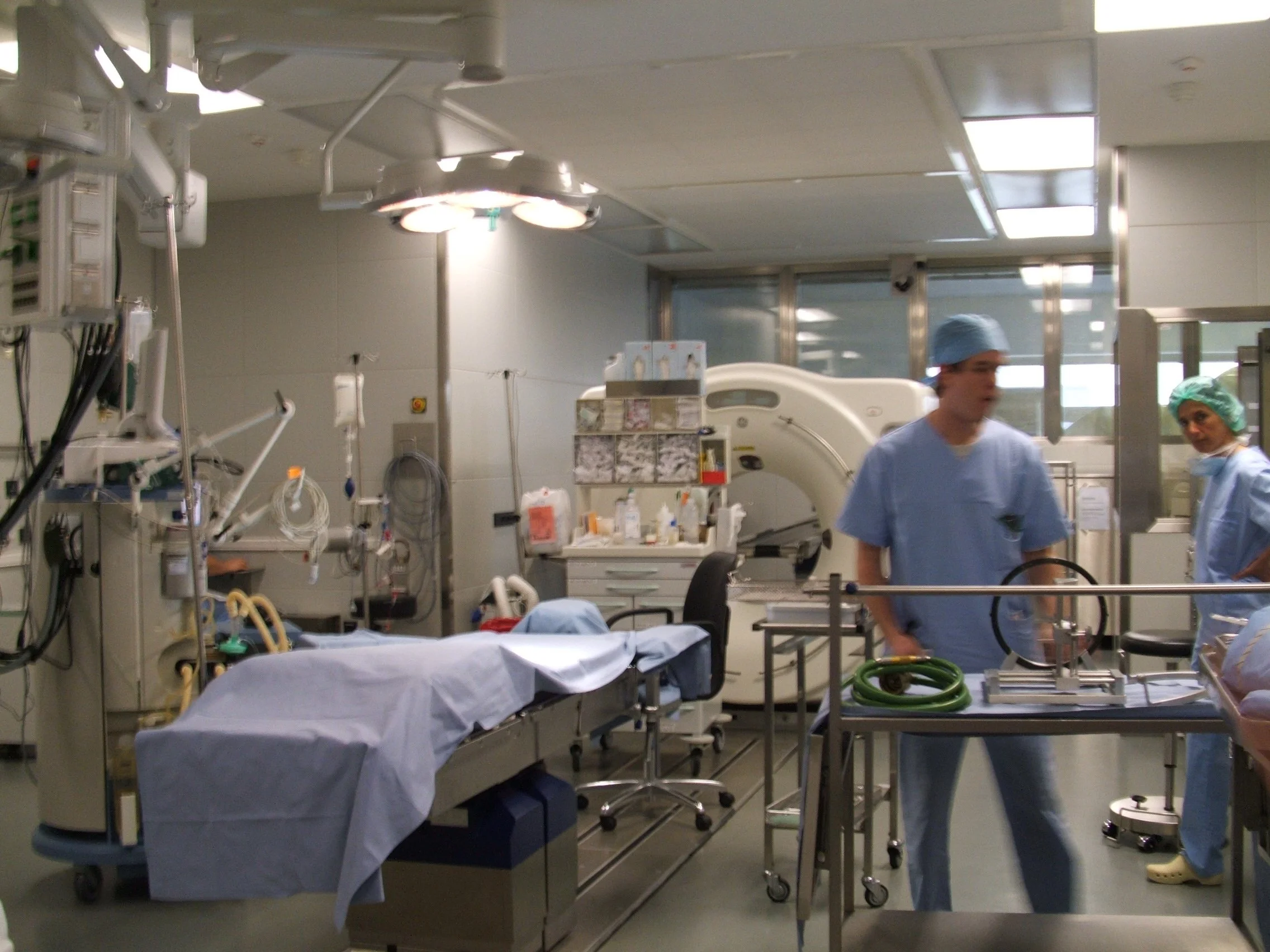
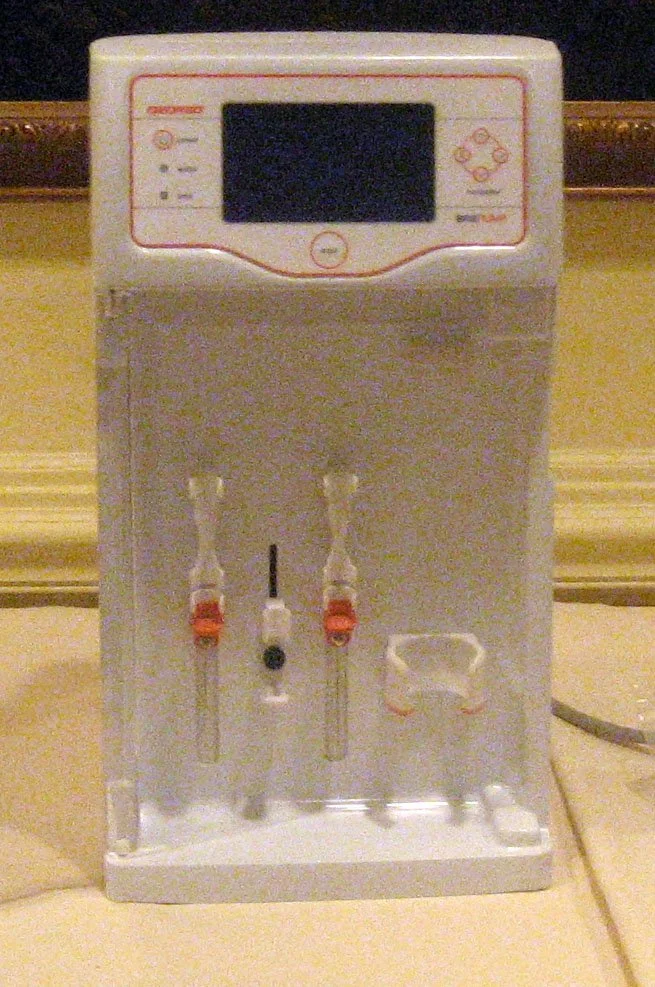
An end-to-end system had to be investigated, designed, and delivered for live patient trials taking place across the globe. It would consist of a Wash System, Administration Device, and Convergence Matrix Disposal.
A key factor of this system was to ensure that it was reliable and repeatable, without fail. The delivery device itself was required to provide adjustable bolus injections and be mounted on a stereotactic-targeting headframe device which was attached to the patient using MRI positioning.
This would be the first of its kind system that would create a device presence in the neurosurgical field for Medrad.
Methods
Alignment workshops across design, hardware and software engineering teams, neurophysiologists, pharmaceutical technicians, neurosurgeons, FDA Regulatory and Clinical Affairs, and global project management teams were critical to the success of this project.
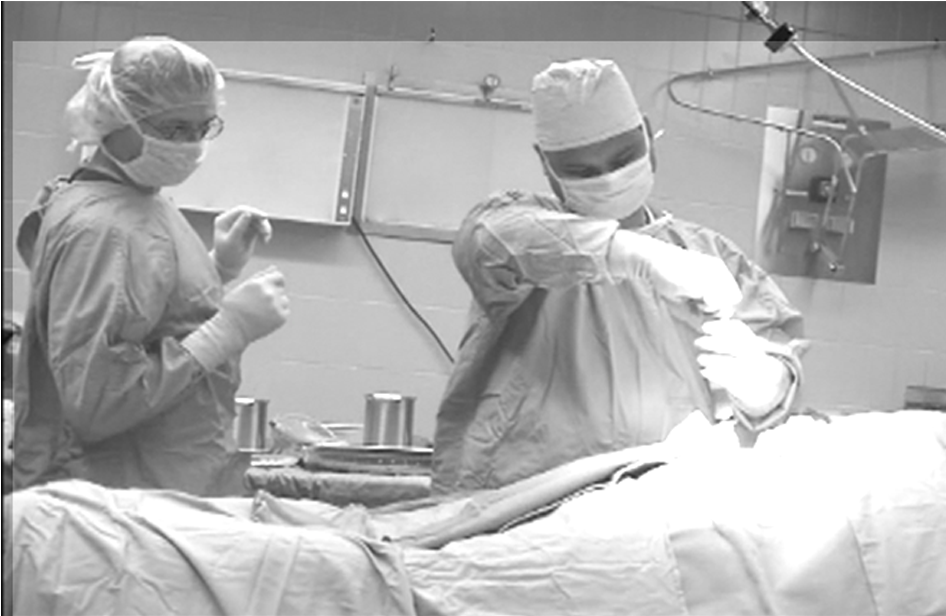
I sat in and observed numerous stereotactic neurological surgeries around the world and conducted immediate post-event interviews with the surgical teams, documenting them in detail for our broader U.S. based innovations team.
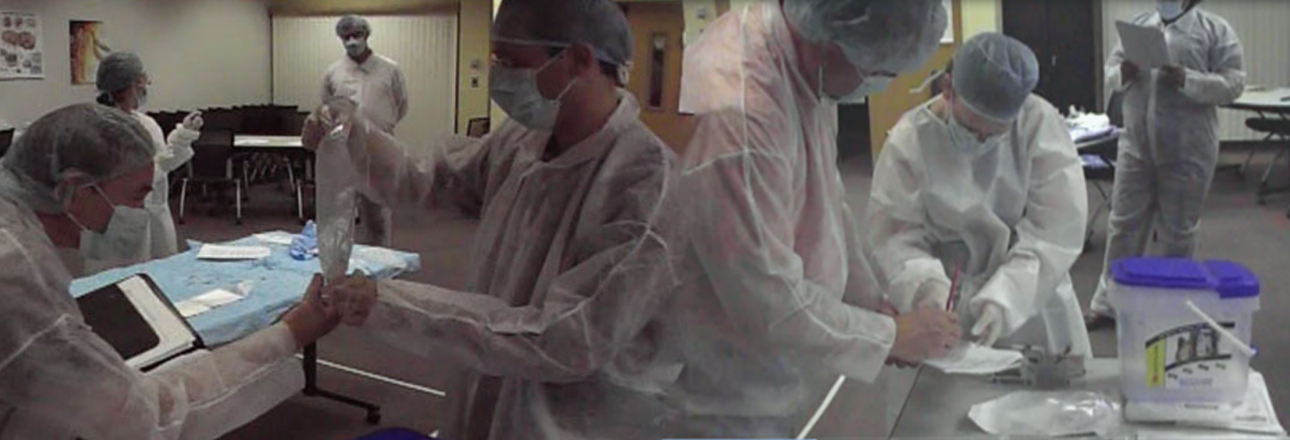
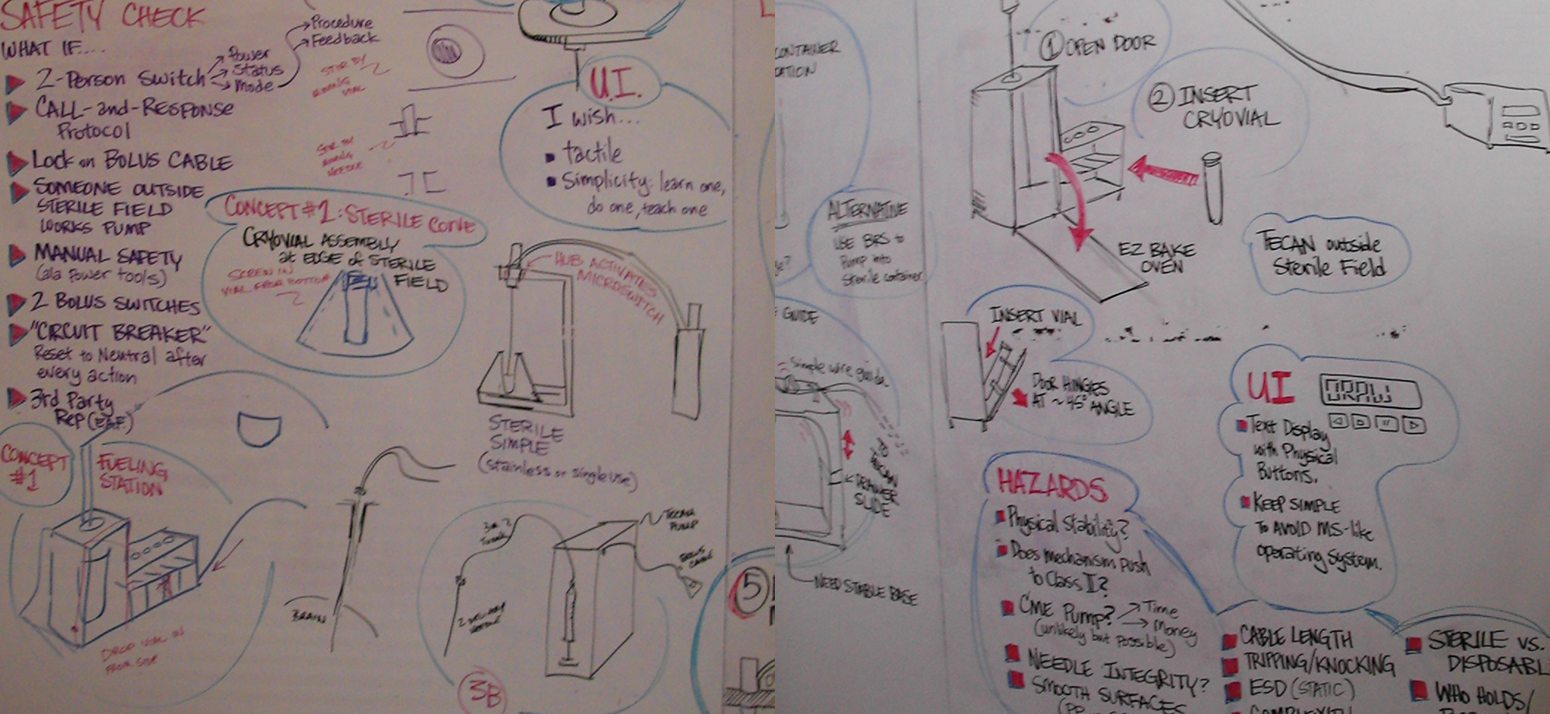
To really create a connection and sense of empathy between the engineers and the surgical teams, I could not rely solely on documentation - I instead designed ‘Mock Surgeries’ complete with ‘scripts’ for the development teams to participate in as well as involving a Surgical Assistant who would stop the participants each time they ‘broke’ protocol or the sterile field. Suddenly, the gravity of the challenges faced by the end users became very real and easier to understand. Once we had a unified understanding of the challenges, risks, and boundaries within which we had to work, I began to conduct multiple ideation sessions across all disciplines involved.
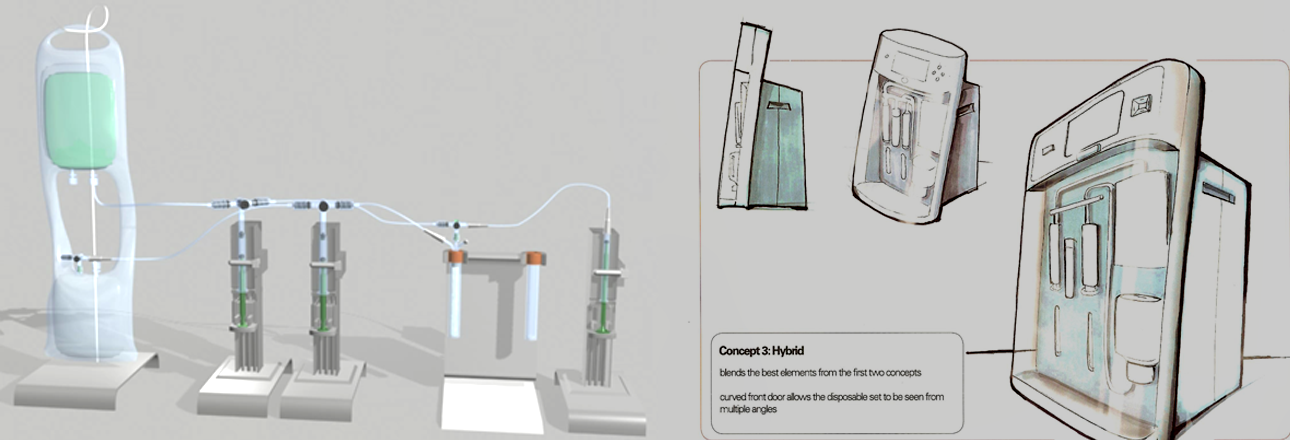
The ideation sessions started off with conceptual brainstorming for each piece of the system which we then narrowed down to three primary concepts. I took these concepts out to the same subject matter experts to conduct comparative testing. After processing these results with the software and hardware teams, we created physical prototypes that could be tested in a simulated environment.
A final end-to-end solution was designed and created, and still used today in the successful treatment of Parkinson’s.
Success Criteria
Address significant ‘knowns’ and identify ‘unknowns’ involving processing, handling and injection procedures
Delivery device interface must be simple to use and compatible with multiple brands of stereotactic headframes
Delivery device should be able to be leveraged in future stem cell delivery therapeutics beyond neurology
Must be a ‘closed system’ to prevent contamination when transported in unsterile environments
Wash system must maintain fluid sterility and be sensitive enough to not damage stem cells in the process
Many Voices Heard
Engaging and gaining buy-in of the quite varied array of voices and participants went well beyond a standard product development team and a set of stakeholders for this groundbreaking therapeutic treatment of Parkinson’s Disease
Meticulous documentation and project coordination was required to meet the standards of the FDA Regulatory Affairs program in order to receive approval to move forward and multiple steps throughout the program - so working with our in-house counsel was an absolute must
A big challenge was creating a relationship and connection between the engineering teams and the surgical staff in order to get them to speak a ‘shared language’ and prevent over talking each other - collaboration was key
Spending time with the patients and their families who were undergoing this completely new experimental procedure, and understanding their hopes and fears, and then relating these conversations back to the innovations team instilled a strong passion for success - it made it less ‘theoretical’ by putting faces to the procedure
Finally, it was imperative to communicate clearly to our international senior stakeholders that the progress milestones were being met, and the value of this investment
Key Highlights
Immersion Workshops, Alignment Workshops, Project Coordination, Subject Matter Experts, Stakeholder Alignment, ProEngineer, FormZ, Rapid Prototyping, Usability Testing, Waterfall Methodology, User Experience Design & Research, User Interface Design, Journey Mapping, Workflow Diagramming, Project Roadmapping, Strategic Market Protection